We're experienced, knowledgable, and connected to ensure quality submissions.
 Drug legislation and regulation play an important role in ensuring the safety and efficacy of approved drugs. Regional agencies regulate the development, pricing, quality, marketing, consumption and most other aspects of commercialization. These agencies require that new and existing products are indeed improving the health status of our growing population. Every country has unique regulations with the aim to maintain the standards of the drug throughout clinical trials while ensuring the best care for patients. Our regulatory team does not just advise you, we represent you with authorities and committees. At PSNResearch, our legal and regulatory experts don’t only consult pre-development, they can fully manage all legal and regulatory processes throughout your study and legally represent you at each stage along the way.
Drug legislation and regulation play an important role in ensuring the safety and efficacy of approved drugs. Regional agencies regulate the development, pricing, quality, marketing, consumption and most other aspects of commercialization. These agencies require that new and existing products are indeed improving the health status of our growing population. Every country has unique regulations with the aim to maintain the standards of the drug throughout clinical trials while ensuring the best care for patients. Our regulatory team does not just advise you, we represent you with authorities and committees. At PSNResearch, our legal and regulatory experts don’t only consult pre-development, they can fully manage all legal and regulatory processes throughout your study and legally represent you at each stage along the way.
We offer a complete range of services as part of a customized package for your program. It will be designed to help you fully comply with regional requirements, including:
Strategy Planning
- Provide legal representation within the US and EU
- Conduct regulatory agency meetings and responses
- Ensure compliance with International Conference of Harmonization (ICH) and Good Clinical Practice (GCP) guidance
- Direct your responses and reactions to FDA and EMA legislation
Briefing Documents
- Liaise with the ethics committees and authorities
- Apply for Investigational New Drug (IND), Investigational Medicinal Product Dossier (IMPD), Clinical Trial Agreements (CTA) and Clinical Trial Exemptions (CTX)
- Submit clinical trial authorizations and amendments
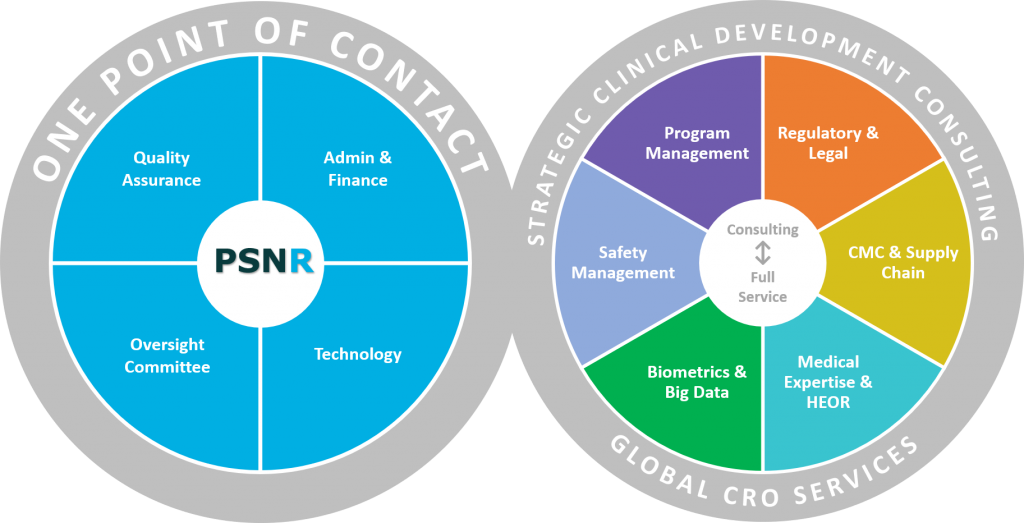
Clinical Trial Services
- Sign contracts between sponsors and sites
- Prepare submissions for FDA, EMA and Institutional Review Board (IBR) review
- Investigator brochures
- Electronic submissions for sponsor and sites
- Dedicated teams for unique and customized requirements
- Translation services
- Local patient insurance
Application Packaging
- Orphan indications and fast track designations
- Write, review, format and submit assessment and technical documents
Commercialization
- Marketing applications, including new drug applications and marketing authorizations Operational support experts in economic and regulatory concerns in the US and EU
 Drug legislation and regulation play an important role in ensuring the safety and efficacy of approved drugs. Regional agencies regulate the development, pricing, quality, marketing, consumption and most other aspects of commercialization. These agencies require that new and existing products are indeed improving the health status of our growing population. Every country has unique regulations with the aim to maintain the standards of the drug throughout clinical trials while ensuring the best care for patients. Our regulatory team does not just advise you, we represent you with authorities and committees. At PSNResearch, our legal and regulatory experts don’t only consult pre-development, they can fully manage all legal and regulatory processes throughout your study and legally represent you at each stage along the way.
Drug legislation and regulation play an important role in ensuring the safety and efficacy of approved drugs. Regional agencies regulate the development, pricing, quality, marketing, consumption and most other aspects of commercialization. These agencies require that new and existing products are indeed improving the health status of our growing population. Every country has unique regulations with the aim to maintain the standards of the drug throughout clinical trials while ensuring the best care for patients. Our regulatory team does not just advise you, we represent you with authorities and committees. At PSNResearch, our legal and regulatory experts don’t only consult pre-development, they can fully manage all legal and regulatory processes throughout your study and legally represent you at each stage along the way.