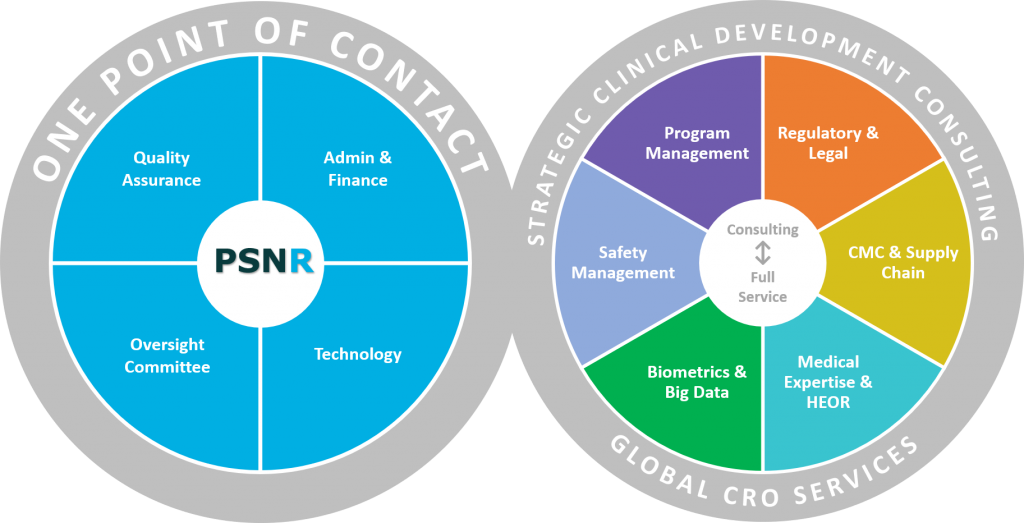
 Given that pre-commercialization approvals make up a substantial portion of overall drug development costs, pharma is intensely focused on ensuring that trials are designed and executed to optimize success. Too frequently, part of the project is forgotten until other parts are concluded. If Chemical, Manufacturing and Controls (CMC) and supply chain are ignored, the start of a trial is significantly prolonged. It’s very important not to overlook the broad nature of CMC and supply chain processes. That’s why PSNResearch has acquired the best experts in this field to address the dynamic predictable and unpredictable supply chain issues that occur during trials.
Given that pre-commercialization approvals make up a substantial portion of overall drug development costs, pharma is intensely focused on ensuring that trials are designed and executed to optimize success. Too frequently, part of the project is forgotten until other parts are concluded. If Chemical, Manufacturing and Controls (CMC) and supply chain are ignored, the start of a trial is significantly prolonged. It’s very important not to overlook the broad nature of CMC and supply chain processes. That’s why PSNResearch has acquired the best experts in this field to address the dynamic predictable and unpredictable supply chain issues that occur during trials.
We carefully consider how go or no-go decisions impact your CMC and supply chain plans. Complex, multi-phase projects involving dozens or hundreds of culturally and geographically diverse stakeholders require that assets and data are collected, cleansed, managed, and analyzed properly. We provide solutions and qualified individuals with the proper accountability for your unique and demanding trials.
Investigational Products
Clinical Trial Supply Chain
Clinical Trial to Commercialization