 Biotech, medical devices manufacturers and pharmaceutical companies develop products that impact patient’s lives – and the lives of family and caretakers. Many drugs have limited impact on efficacy and safety versus drugs already on the market. As we evolve as a society of drug developers and caretakers, we want more for patients. That’s why basic medical expertise, plus advanced therapeutic expertise are so important. Breakthrough treatments for illnesses are more and more grounded in advanced research.
Biotech, medical devices manufacturers and pharmaceutical companies develop products that impact patient’s lives – and the lives of family and caretakers. Many drugs have limited impact on efficacy and safety versus drugs already on the market. As we evolve as a society of drug developers and caretakers, we want more for patients. That’s why basic medical expertise, plus advanced therapeutic expertise are so important. Breakthrough treatments for illnesses are more and more grounded in advanced research.
Our team is made up of 400+ innovative and engaging healthcare professionals with senior management experience in pharma. Advanced therapy medical products require special preparation and review of clinical documentation, advanced management of orphan drug designation applications to the FDA and EMA and extra support on the design of early clinical trials and efficient operations.
And now, first-in-human (FIH) studies have more deviations, such as patients receiving a completely novel drug or a combination of new and approved drugs. Or specific patients being exposed, such as the elderly. PSNResearch has devoted considerable time and resources to the development of a proprietary process model specifically tailored to FIH studies.
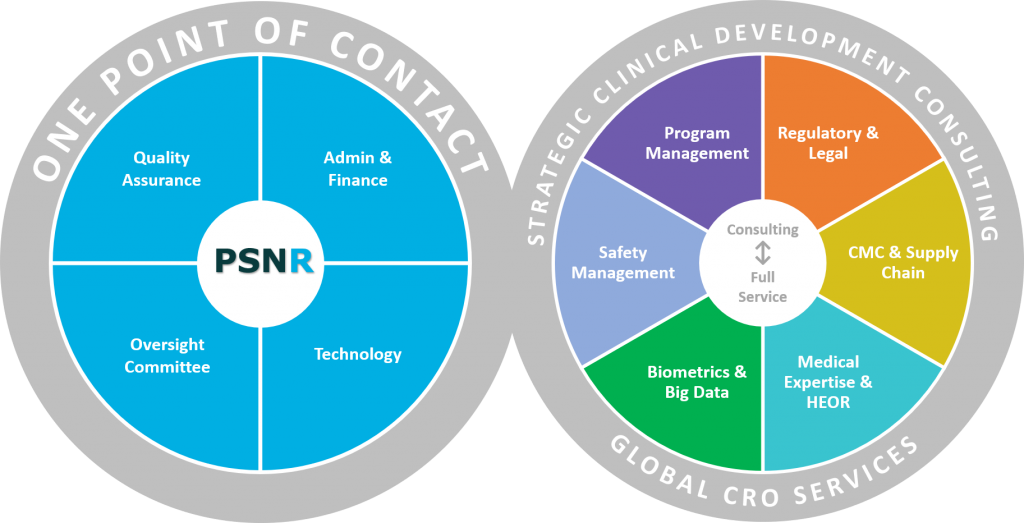
Medical advisors located in eight countries consult on your clinical development plan:
We use unique and adaptive approaches to clinical research services. We know how to identify biomarkers to determine clinical endpoints, as early in the clinical process as possible, to ensure successful ongoing protocol design.
Medical Writing
Medical Documents
Investigator and Patient