Six medicines recommended for approval, including one orphan
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) recommended six medicines for approva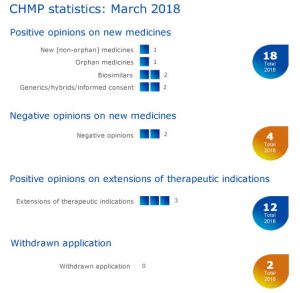 l, including one orphan medicine1, at its March 2018 meeting.
l, including one orphan medicine1, at its March 2018 meeting.
- The CHMP recommended granting a conditional marketing authorisation for Rubraca(rucaparib), for the treatment of relapsed or progressive ovarian cancer.
- Juluca (dolutegravir / rilpivirine) received a positive opinion for the treatment of human immunodeficiency virus (HIV) infection.
- Biosimilars: Kanjinti (trastuzumab) for the treatment of breast and gastric cancer; and Zessly (infliximab) for the treatment of rheumatoid arthritis, Crohn’s disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and psoriasis.
- Generics: received a positive opinion from the CHMP: Pemetrexed Krka(pemetrexed), for the treatment of malignant pleural mesothelioma and non-small cell lung cancer; and Prasugrel Mylan (prasugrel), for the prevention of atherothrombotic events.
Negative opinions on two new medicines
- Dexxience (betrixaban). Dexxience was expected to be used for the prevention of venous thromboembolism.
- The Committee also adopted a negative opinion for Eladynos (abaloparatide). Eladynos was intended to be used to treat osteoporosis.
Three recommendations on extensions of therapeutic indication
The Committee recommended extensions of indications for Cabometyx, Ivemend and Repatha.
Start of re-examination of recommendation on extension of therapeutic indication
The applicant for Sutent (sunitinib) has requested a re-examination of the Committee’s negative opinion for this medicine adopted at the February 2018 meeting.
Start of referral: omega-3 fatty acid medicines
The CHMP started a review of the use of omega-3 fatty acid medicines in patients who have had a heart attack, following research showing that these oral products may not prevent recurrence of heart disease or stroke.



