The EU PAS Register was launched in November 2010 as unique source of information on the safety and effectiveness of authorised medicines. This platform is openly and accessible which includes information on observational post-authorisation research in medicines already marketed in Europe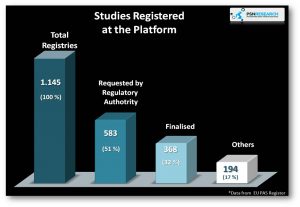
and some infor that includes is:
- Study protocols
- Study results
- Related publications
Data from 31st f July 2017 – EU PAS Register Platform
All the information at the EU PAS Register helps to reduce publication bias through increased transparency of medicines research, improves the quality of post-authorisation studies by facilitating peer-review of protocols and results, promotes collaboration among stakeholders, and ensures compliance with EU pharmacovigilance legislation requirements.
The platform is recommended in the following cases: scientific publications, guidelines and textbooks. Although initially the main aim of The EU PAS Register was to collect studies conducted in the European Union, researchers from outside the EU are also registering studies to increase transparency of their research.
The EU PAS Register was developed through the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP), which is coordinated by EMA to support research in pharmacoepidemiology and pharmacovigilance.
If you want to consult more info abour EU PAS Register Platform, please click on the link below:



